We're Off To See The Wizard - Storing Energy Using Ammonia
Posted by Big Gav on April 29, 2008 - 6:01pm in The Oil Drum: Australia/New Zealand
There were a couple of small Australian solar power projects that I left out of my look at solar thermal power a little while ago, as I thought they were worthy of separate consideration.
The first of these is being put together by a South Australian company called Wizard Power, which is trying to commercialise research from the Australian National University (ANU) - a solar concentrator dish and a closed loop thermochemical energy storage system using ammonia.
Most solar thermal projects use molten salt or water to store energy in the form of heat, as will another Australian solar thermal plant that uses graphite as the storage medium.
Like other solar thermal companies (Ausra being the highest profile example), Wizard is touting the coupling of energy storage with solar power as a "baseload power" solution, with the goal for the new plant being to provide power 24 hours a day to the South Australian town of Whyalla.
 Wizard plans to start construction of a demonstration plant in October and to begin generating power from July 2009. Six concentrator dishes will be built on land opposite the OneSteel steelworks, which receives an average of 301 days of sunshine each year.
Wizard plans to start construction of a demonstration plant in October and to begin generating power from July 2009. Six concentrator dishes will be built on land opposite the OneSteel steelworks, which receives an average of 301 days of sunshine each year.
The demonstration plant was funded with an Australian Greenhouse Office grant of $7.4 million as part of the Advanced Electricity Storage Technologies programme, and the plant may be expanded if the technology is proven.
There have been reports that an associated company, Wizard IT, has gone into receivership, but apparently this does not impact the Wizard Power organisation.
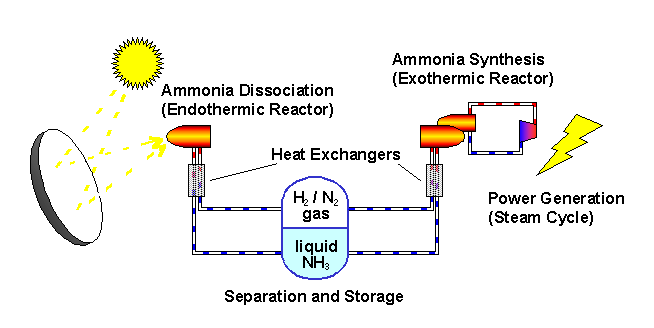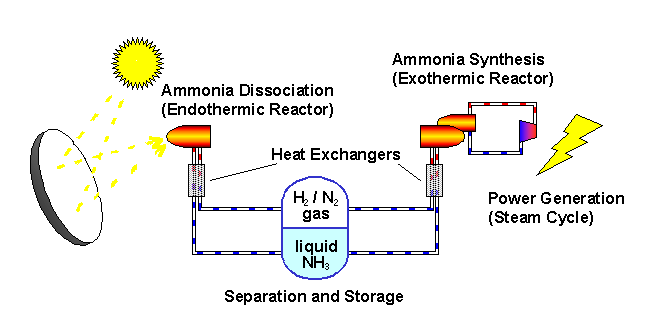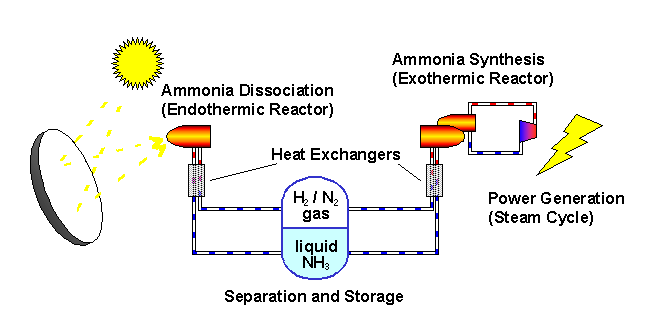
The ANU describes the "closed loop thermochemical energy storage using ammonia" process as :
n this system, ammonia (NH3) is dissociated in an energy storing (endothermic) chemical reactor as it absorbs solar thermal energy. At a later time and place, the reaction products hydrogen (H2) and nitrogen (N2) react in an energy releasing (exothermic) reactor to resynthesise ammonia.
2 NH3 + Heat <---> N2 + 3 H2
A fixed amount of reactants (ammonia, nitrogen and hydrogen) are contained in a closed loop, and pass alternately between energy storing and energy releasing reactors with provision for storage of reactants in between. Because the solar energy is stored in a chemical form at ambient temperature, there are zero energy losses in the store regardless of the length of time that the reactants remain in storage. The reactors are packed with standard commercial catalyst materials to promote both reactions. Counter-flow heat exchangers transfer heat between in-going and out-going reactants at each reactor to use the energy most effectively.
Feeding the reactors with pure reactants is possible through the natural separation of reactants and products in the storage system: at the pressures applied, ammonia condenses.
By ensuring that the stuff leaving each reactor transfers its own thermal energy (sensible heat) to the stuff going in - using heat exchangers - most of the solar energy is stored in the change in composition of the chemicals which are kept at ambient temperature.
The advantages of this energy storage mechanism are identified as:
* A high energy storage density, by volume and mass.
* The reactions are easy to control and to reverse and there are no unwanted side reactions.
* All constituents involved are environmentally benign.
* There exists a history of industrial application with the associated available expertise and hardware.
* A readily achievable turning temperature of 400oC to 500oC (depending on the pressure). This helps to reduce thermal losses from dish receivers, avoids some high temperature materials limitations, and allows lower quality (and hence cheaper) dish optics to be used.
* All reactants for transport and handling are in the fluid phase, which provides a convenient means of energy transport without thermal loss.
* At ambient temperature the ammonia component of reactant mixtures condenses to form a liquid, whilst the nitrogen and hydrogen remains as a gas. This means that only one storage vessel is required for reactants and products.
There is also a possibility of using the low grade heat left after power generation for secondary applications such as desalination.
I haven't been able to find any comparisons of thermochemical energy storage to other storage mechanisms, so its hard to e certain how much of a boost (if any) this process gives compared to the alternatives.
Dr Keith Lovegrove from ANU mentioned some details regarding the solar dishes used in a talk on "Concentrating On Solar Thermal as a Solution to Climate Change" at the "Zero Emission" Conference in Melbourne last year.
A quick comment on why ANU advocates dishes, rather than other alternatives. Essentially if you go through the numbers, we pick up a higher optical efficiency and higher thermal efficiency in the receiver and that also propagates through. Our turbines will be the same as anyone else's turbines but at the end of the day, we think we'll get twice the electrical output per area of mirror. So that's just in case you're following that route. ...
Where is solar thermal power going? I think we can learn from the wind industry. It’s very similar. It's about manufacturing, the use of steel and glass and not rocket science. Wind industry has grown exponentially and costs have declined. And we can expect the same. ...
Here's a thought. We could actually export solar energy. How would we do that? Well, we would do that by using, for example, solar thermal systems can gasify biomass and even, dare I say it, gasified coal, in which case the final energy content is a mixture of solar and fossils. You can synthesise all that stuff into methanol and ship it overseas and quite literally power Japan, given that they're 40% dependent on Middle Eastern oil at the moment and not very happy about that. It's quite conceivable to imagine Australia as an exporter of solar energy.
I'll close with a quote from Thomas Edison back in 1910 (Source: Interview in Elbert Hubbard's Little Journeys to the Homes of the Great):
Some day some fellow will invent a way of concentrating and storing up sunshine to use instead of this old, absurd Prometheus scheme of fire. I'll do the trick myself if some one else doesn't get at it. Why, that is all there is about my work in electricity--you know, I never claimed to have invented electricity--that is a campaign lie--nail it!
Sunshine is spread out thin and so is electricity. Perhaps they are the same, but we will take that up later. Now the trick was, you see, to concentrate the juice and liberate it as you needed it. The old-fashioned way inaugurated by Jove, of letting it off in a clap of thunder, is dangerous, disconcerting and wasteful. It doesn't fetch up anywhere. My task was to subdivide the current and use it in a great number of little lights, and to do this I had to store it. And we haven't really found out how to store it yet and let it off real easy-like and cheap. Why, we have just begun to commence to get ready to find out about electricity. This scheme of combustion to get power makes me sick to think of--it is so wasteful. It is just the old, foolish Prometheus idea, and the father of Prometheus was a baboon."
When we learn how to store electricity, we will cease being apes ourselves; until then we are tailless orangutans. You see, we should utilize natural forces and thus get all of our power. Sunshine is a form of energy, and the winds and the tides are manifestations of energy. Do we use them? Oh, no! We burn up wood and coal, as renters burn up the front fence for fuel. We live like squatters, not as if we owned the property.
There must surely come a time when heat and power will be stored in unlimited quantities in every community, all gathered by natural forces. Electricity ought to be as cheap as oxygen, for it can not be destroyed. Now, I am not sure but that my new storage-battery is the thing. I'd tell you about that, but I don't want to bore you...
Cross-posted from Peak Energy.

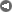


Some earlier ammonia discussion.
How shall driving gain nuclear cachet?
Yeah - I thought about using some of that material in this post but decided it needed a post of its own, as its not really related to the energy storage technique used by this project.
Excellent post. That ammonia storage process really looks like quite something. Exporting solar energy? Now that's something I'd love to see. Although I think we'd be back to square 1 with OPEC ;).
The process was patented in 1989. See US patent 4829768, Fluid dissociation solar energy collection system, Carden, Peter O. Assignee: Anutech Pty. Ltd., Canberra, Australia.
Most easily available at Google Advanced Patent Search:
http://www.google.com/patents?q=&btnG=Search+Patents
It's not the same process. The process in the patent expands the dissociated product stream in a turbine to make work. The process described here recombines the dissociated products in a reactor to make steam. The patent is by the research group at ANU. It contains references to their earlier work.
This project has particular significance as Whyalla will be in the energy spotlight. BHP Billiton has said they want 690 MW for the Olympic Dam expansion presumably including a desal plant on the nearby coast. Other new mines (eg Prominent Hill) are planning transmission lines to Roxby Downs.
OneSteel has coke ovens supplied by coal ships from Newcastle. Water and gas pipelines run down the coast, water from the River Murray and gas from Cooper Basin. Santos has a gas fractionation plant about 30km out of Whyalla where LNG ships call in once fortnight I believe, until Cooper Basin runs out.
The linked news story doesn't give an electrical output for the CSP plant, but if it supplies 10,000 homes I'll infer that as 10 MW. While that seems large it is infinitesmal compared to other captured energy flows associated with the area. The article doesn't state how long this plant can maintain output in overcast weather. To me 'baseload' means the ability to supply a specified output 24/7, barring unplanned outages.
So I see this project as a David and Goliath battle of small scale renewable versus the fossil and nuclear juggernaut.
Regarding size, remember - its just a demonstration plant to trial the technology commercially.
I agree that there is no detail about storage capacity - I'm hoping someone with some more knowledge will comment at some stage.
I view the renewables vs extraction based energy sources battle as a mammals vs dinosaurs one rather than a Dvid vs Goliath one.
And unlike those Goliath's, this one could be quite comfortably distributed in the suburbs... potentially generating (most of) that residential baseload where it's needed.
Remember that the first nuclear reactor was essentially a pile of uranium covered by some graphite bricks that produced "zero" power.
And the first coal fired engines don't really stack up today either...
How many horse power was "The Rocket"?
Here is a technology with ZERO emissions, scalable, that uses well understood standard processes AND we don't have to wait for "carbon capture and storage"... I hope the trial is successful.
Anyways... David won!
Nice post Gav.
G'day mates, any chance I could have a little piece of the South Florida franchise? I promise to put a few large shrimp on the barbie for you if you come over for a visit. I'll even throw in a tour of our coral reefs.
"Here is a technology with ZERO emissions, scalable, that uses well understood standard processes AND .."
Well, just like Dr. Lovegrove points out above,
Setting it up and transport are the real real problems.
Meaning that Edison was right all the way. It has to do with breaking the bariers of collecting diffuse energy AND storing it. Right now, collecting is a tick too expensive and storing/transporting it breaks the bank. I've started the Ammonia discussion in other threads and think it will be ONE very good opportunity to store/use solar and wind being produced in Australia or the Sahara/MidEast. There are many brains working on the "problem" right now: http://www.desertec.org/ .
I have a greatly improved way to transport it, but you'll have to wait til I start my own company and publish the patents for that.
Anyone want to give me a couple million start capital for it?
All the best, Dom
SP enthuses that the technology is "scalable" but Big Gav, the author, says "I agree that there is no detail about storage capacity". So I think SP is premature. Technology like this has been around for quite a while and has not gone anywhere. There is probably a reason.
Let's hope we can find some real, scalable solutions to the energy storage need.
What is the reason?
Yes, I am probably premature in my enthusing, and I should have used the appropriate modifier 'potentially'. But we are talking about a trial.
The argument that becuase "Technology like this has been around for quite a while and has not gone anywhere" ... can be used to dismiss anything.
Battery Technology.
Cancer research.
Fusion.
I'm pretty excited about this method because the dishes seem to build on work that was carried out in astronomy in Australia by Hanbury-Brown and Twiss. They measured the sizes of stars using a novel form of interferometer called the intensity interferometer built near Narrabri in the 1960s.
I'm hopeful these facilities will be used in a similar way at night once they get going.
Chris
These new facilities will never be used for astronomy for a the very simple reason of cost-effectiveness, which in this case means poor mirror quality. For passive solar, it a focus of a few centimeters in diameter should be good enough, while for optical astronomy, the mirrors have to have a perfect shape down to the order of magnitude of some tens or hundred nanometers. It would be a huge waste of money to build perfect mirrors for heat generation.
That was kind of the point with the intensity interferometer. Tens of arcminutes resolution was fine. Thus, they could use the segmented mirrors you see in the image long before the active control used in the Keck. The intensity interferometer is much less sensitive than the Michelson interferometer in terms of collecting area which is why they used such large mirrors to work on bright stars. But, it does not need the extreme tollerances of a Michelson. Much more importantly, recorded signals can be used to to produce the interference and so the senstitivity of the intensity interferometer (in terms of the amount of collecting area) exceeds that of the Michelson when there are approximately 100 or so stations. This is because the number of beams that can be combined in a Michelson is limited to less than ten or so, while all possible baselines can be measured with the intensity interferometer. This crossover figure includes some methods I've developed to improve the sensitivity of the original Hanbury-Brown and Twiss approach on a single baseline. One of the truly fundemental next steps in astronomy is to measure the rotational orientation of stars. An intensity interferometer array is particularly well suited to this since it has both spatial and Fourrier transform capability so that it is possible to measure the changing doppler shift of a spectral line across the face of a star. If you want to do work that will be included in the introductory text books, this would be it. The science that can be accomplished from an archeology of angualar momentum in clusters of stars is breathtaking, yielding perhaps the most intimate details of star and plant formation that are possible to discover.
Dish solar collectors that are built to achive fairly high temperture are essentially perfect intensity interferometer base stations for night time use and only slight additions are needed to instrument them for this purpose which can be configured not to cause any difficulties with the main goal of collecting solar energy. By contrast, configuring arrays of solar panels for all sky monitoring and intensity interferometry may require intrusive modification of the electonic design and could be much less feasible. Night time use of panels may rather concentrate on transient detection and perhaps study of ultrahigh energy cosmic rays.
If you have access to a library that has a copy of Hanbury-Brown's book on the intensity interferometer I recommend it as one of the best written books in science.
Chris
A dumb question: Where does the ammonia (NH3) come from?
Bullshit ;-))
Ammonia is in plentiful supply (for the time being) and the system is a closed loop - the amount required to run the system is likely to be inconsequential compared to that required for fertiliser (farming) or explosives (mining) in the region.
Ammonia (NH3) is made via Haber synthesis from H2 and N2. Nitrogen can be fractionally distilled from air, and renewable hydrogen can be produced by methods as diverse as electrolysis of water and oxidation of charcoal with steam.
There are some questions I have about this post.
First, ammonia is a poison to fauna, so will it be environmentally benign in case of rupture?
Second, the storage medium is two gases; will that not require an enormous container to hold the substantial volume?
Third, hydrogen being what it is, will there not be a necessity for extra special materials to avoid losses thereof?
Yes, Ammonia in "high" concentrations, will kill you.
CO2, in "high" concentrations, will kill you.
N2, in "high" concentrations, will kill you.
Apples, in "high" concentrations, will kill you.
Don't get hung up on it.
In the event of a rupture, don't stand nearby. It is unlikely to be a catastrophic without warning rupture though. And being ammonia, small leaks will be readily detected by the remarkable sensor at the end of our faces... in addition to electronic sensors deployed to monitor the plant. IE in all probability, there will be warning.
In the event of a leak, the dispersed ammonia will become absorbed by water and adsorbed to soil. It will be "rapidly" utilised by bacteria and plants.
Not too many years ago, before we discovered chloro-fluoro carbons (ie freon), refrigeration used ammonia.
The ammonia is the storage medium. It is recycled and only needs to be synthesised once. In fact it is continuously being disassociated and reformed.
Gases have a useful property... they can be compressed. If you visualise an incredibly large centralised system then yes, the storage vessel would be large (it could be buried). But a large system may not necessarily be the best way to deploy this technology. It's still a trial.
Unlike, "the hydrogen economy tm", the requirement for "extra special materials" would be minimised. Unlike "the hydrogen economy" there is no requirement for cars, houses and pipelines to each have hydrogen storage mechanisms.
Unlike, the hydrogen economy, lost hydrogen in this system could be made up for by H2 derived from water. IE there need not be any requirement for H2 derived from natural gas.
There is no perfect "solution".
The thoughts above are only my un-researched, non-internet linked opinions derived essentially from my general reading.
Thanks for nothing SP! None of your comments showed any thought about the meanings of the questions nor about the actual process.
If you reread the actual description, ammonia is the feed stock and the N2 / H2 pair are the storage medium. So yes, the storage of these gases will require substantial vessels built of special materials to hold the hydrogen. I made no mention of a hydrogen economy, I was smply questioning being able to store hydrogen for long periods of time under any circumstances except perhaps in special containers? Your suggestion of making more hydrogen from water smacks of perpetual motion machine [use the energy from the generator to power electrolysis to split water].
As far as the benign qualities of ammonia, it is not as bad as other gases I'm sure, but in the quantities required for large scale generation, a rupture could release sufficient amount to maim and even kill.
Actually, I put some effort (after my initial, I thought light hearted, sarcasm) into that comment.
All three gases are stored in the same container!
It was actually step backs comment, and also yours about storage of hydrogen, that made me mention the hydrogen economy; storage of H2 being one of the technical challenges for widespread distribution of H2 as an energy carrier.
You describe ammonia as the "feed stock" and step back also made a comment that implies that this is a once thru process.
This is not a once thru process.
The ammonia is not consumed but recycled. Look at the picture.
The sunlight provides the heat to drive the reaction one way... we recover some of the heat when the reaction is reversed.
In the event of small H2 leaks, onsite generation of H2 from electrolysis of water would be a practical solution to "top up" the hydrogen supply and not as you bizarrely claim some kind of "perpetual motion" mechanism - an idea stemming from your mistaken belief that this is a once thru process.
As to your concern about ammonia...my point was simply... yes there is a risk, but its not that great. This is a material that is widely used in many chemical and manufacturing processes. It's used by farmers. That is one of the benefits of using ammonia. It's behaviour and effects are understood.
Now, I've spent about another 40 minutes on this reply. I hope it helps you understand the process better.
The answer is yes - you do need a storage vessel to hold the Nitrogen and Hydrogen gas.
How large the vessel is and what pressure the gas is held at isn't clear from any of the material I've been able to find.
However, storing hydrogen isn't rocket science and is done at many other locations, so I doubt it presents an insurmountable challenge.
The question is how much gas storage do you need to store "energy" that can be released later - ie. what size / strength vessel is needed to store enough gas to produce 16 hours worth of generation at 10 MW (in the case of this plant) - and I haven't seen a hint of what the answer is to this...
However, storing hydrogen isn't rocket science
And here I thought Liquid H2 was a big part of the science of rockets!
it presents an insurmountable challenge.
Depends on how one defines 'insurmountable'. As I understand, at the higher pressures, H2 leaks at about 5% a day. So the closed loop system strikes me as the frictionless, point mass bodies - a fine theory but the physical realities makes sure things just don't work that way.
(The interesting set of calcs would be how often the system could loose all of its gasses due to accident and still be making energy.)
OK, so make that "storing hydrogen as a gas is not rocket science ... that's storing hydrogen as a liquid".
Of course, hydrogen is just about the trickiest gas there is when it comes to leaks, which is one reason why ammonium may be a more sensible transport medium than hydrogen for direct conversion of, say, windpower to liquid fuel ... but a closed loop is less challenging to keep sealed than a transport chain with several transfer steps.
You understand wrong. H2 can be held for decades inside steel tubing; the Electrolux-cycle gas-fired absorption refrigerators used in RVs are proof positive.
Well, it's going to be stored around 200 atmospheres because that's where the Haber process works. Similarly the disassociation reactor is probably going to be run near atmospheric pressure. There will be compressors and expanders not shown in the simplified flowsheet.
Gas receivers even for hydrogen are an old technology. Any losses in the cycle would happen in the seals for any rotating equipment.
Methinks you're wrong. Consider the dissociation reactor alone; the energy involved in pumping the 2x increased volume of gas up to storage pressure would seriously cut the net energy output of the system.
Pressure only affects the equilibrium constant for a given temperature, not the change in energy. If the dissociation reactor doesn't crack as much of the NH3 at 200 bar as it does at 1 bar, so what? The un-cracked ammonia yields its heat to the input fluid, and gets cycled again.
The big expense for this process is going to be pressure vessels for storing gas mixtures. I suspect that the answer is going to be steel-lined caverns mined out of deep rock, like the hot-water solar thermal storage concept.
Maybe, maybe not. You'd use an ammonia expander in front of the dissociator direct coupled to a booster compressor to recover a lot of that energy if you went the small reactor route. Most commercial dissociators run at near atmospheric pressure so if you want to use off the shelf units that's where you're running.
Why would the properties of a commercial ammonia dissociator be relevant? This system stores all its stocks in a single tank; if the efficiency of dissociation is relatively low, this only affects the energy recovery in the heat exchangers. The system doesn't require a pure output. Eliminating compressors is going to be a far bigger gain.
Actually, if the dissociation reactors can be run at very high pressure, the dissociation system could be run as a Rankine engine (liquid NH3 in, gases out) and produce net energy from the drop back to storage pressure. That could be the daytime peaking generation, with the recombination supplying the night-time base load.
I found his response to be technically accurate and on target to the literal meaning of your question. If there is any fault regarding failure to satisfy you, it appears to be yours.
Nice animated GIF. That kind of a visual, which is quite unassuming IMO, is something you can't get from a textbook.
Yeah - I thought they did a good job on making it very simple to understand (though obviously it didn't succeed with everyone)...
頑張って下さい。
名古屋 風俗
For anyone who can't read Japanese, that's just a spam bot.
Wait, I thought that ammonia was the high-energy state (seeing as you can burn it). Now they're saying that it takes energy to disassociate ammonia? I thought that disassociating ammonia would release energy, and making ammonia would require energy...
Or maybe this is how it works:
6H20 + energy --> 6H2 + 3O2
6H2 + 2N2 --> 4NH3 + energy
4NH3 + energy --> 6H2 + 2N2
Net reaction: 6H20 --> 6H2 + 302
So this is basically a way to store sunlight that goes beyond simply storing hydrogen (which is difficult to store) and oxygen, but which take the hydrogen and reacts it with atmospheric methane to produce ammonia, which can be easily stored. Right?
Your "equations" contradict your statement...
I think we can trust one hundred years of chemistry since Haber and Bosch, that the fundamental processes and energies are pretty well understood for this reaction...
That's not a valid argument, you can burn H2 as well.
Lots of action in this area - Dr. John Holbrook has an active project to trickle charge ammonia from a hydro facility in order to use it to replace a natural gas peaker. I just stepped out of a meeting with a lobbyist and a similar plan is going in front of Duval Patrick, governor of Massachusetts, on the 12th of next month. Another thing happened today along these lines but it's high level and touchy ... not sure if I can say anything about it yet, or if I have to wait for the big heads to bless & reveal.
Oh, and this crapping solid state ammonia synthesis pilot plant grant is open in another window and the deadline is tomorrow.
Good times here in renewable energy land ... but we do need more money to apply to the problem.
Wouldn't it be more efficient to install an extra turbine at the hydroelectric facility? Or is there some restriction requiring a constant flow rate?
Nope, the need is for 10MW instant on summer cooling, lots of turbines needed for that and the rest of the time they're idle. I don't know all of the details - there is no idle ammonia production where I live :-)
Here's a strange thing, the uranium mines use a huge amount of ammonia
from the UIC website. No mention of leakages from this process. The connection is that Whyalla is the nearest port that has strong sea currents. Also if Olympic Dam mine is to open up to 4 X 3.5 X 1 (kilometres that is) then they will need a lot of explosives such as ammonium nitrate. However they also need .7 GW for mine power and water desalination, about half the output of the State coal and gas fired grid. Some have suggested a 1000MW nuke right next to where this solar plant is proposed.
Thus the solar ammonia plant looks to me like a hamster caught up in an elephant stampede. Still it could all turn around with the hamster outrunning everybody.
As noted by several above, its a pilot plant.
And in precisely these terms:
... a very promising pilot plant, at over 1% of the incremental energy consumption in this area, therefore only needing to scale up 10-fold to be more than 10% of the increment ... and that's what we need. A variety of sustainable energy sources that each can take up more than 10% of energy demands. Incremental energy demands at first, and then as the broader technology ... social organization, financing, etc. ... is hammered out, moving toward total energy demands.
I am not an expert but IMO Ammonia offers a better route to a solution of Energy storage than Hydrogen from what I have read up about the liquid. It has many advantages over Hydrogen:
-I'm sure there are more and would like to see further TOD articles on the subject. (I understand that the process above is unlikely to have any major impact for decades even if scaled rapidly btw. having been lurking here a while)
Nick.
Leakages are reduced
From my looking at H2 as a storage medium was a 5% a day leakage rate. So storing some H with a N - not a bad way of getting H about.
Hi HI Big Gav. Thanks for this post. Great work. Two years ago I saw a (secret) plan for this system that included a geothermal loop. Excess production (sunny days) were marked as "over production" to be sold on. There were even capture/holding tanks for the collection of condensed water that formed on some parts of the infrastructure.
Thanks for the feedback - sounds like an interesting idea coupling geothermal with solar thermal - makes using it as baseload power a lot easier.
Capturing some additional fresh water wouldn't hurt either in that part of the world...
I guess the first question that comes to my mind is to ask what the efficiency of the two reactors are, and what the costs are? Or to put it a different way, how do the costs differ for the ammonia based system and a molten salt system which stores heat instead?
If I remember my high school chemistry, the combination of nitrogen and hydrogen to produce ammonia was one of the great challenges of inorganic chemists. This is because the reaction is highly ENDOTHERMIC not exothermic. Thus there is a FATAL FLAW in the process suggested (described above).
Germany was in dire need of nitrates for explosives during world war I and Fritz Haber developed a method for synthesis of ammonia from hydrogen and nitrogen (http://en.wikipedia.org/wiki/Haber_process)and then a process was developed to convert ammonia to nitric acid, used in manufacture of explosives. Thus for the first time a method was available to "fix" nitrogen chemically. He received a Nobel prize for this process for synthesizing ammonia.
This process is now the basis for producing fertilizers and as noted above makes possible the synthesis of many explosives in large quantities. HOWEVER, the produce demands large amounts of energy and the process is an equilibrium that only produces 15% ammonia in a single cycle. Thus it could not be used in the cycle described in this posting.
IMO it is not as simple as the diagram suggests - remember, they are talking about using the power from windmills to make ammonia in the USA! The point is, ammonia can be easily stored until it is burned to produce power.
Clue number 1. Here they are talking about using power from the sun to break down ammonia.
Clue number 2. Haber-Bosch works at ~200 atmosphere pressure and ~400 degrees C at those temperatures and pressure ammonia is NOT the benign substance claimed and I'm pretty sure you would have to keep topping up the hydrogen.
Clue 3. It is relatively easy to store a liquid like ammonia, very difficult to store large quantites of high pressure high temperature gas.
I am sure overall that the manufacture of ammonia requires energy (it's endothermic) otherwise we are saved since there is no problem powering power stations, just make ammonia - let some hydrogen and nitrogen mix and step well back.
IMO nothing to see here, except maybe crooks, or maybe you have put up a joke posting!
I can think of ways of making ammonia from solar power (which would make some sense) but this isn't one of them.
Except the ammonia is not stored at such temperatures; it, and the gas mixture with which it is exchanged, are stored at near-ambient. This eliminates storage losses from conduction of heat.
Manufacture of NH3 from H2 and N2 is exothermic. Just look at the ΔHf; it's negative, giving off heat (and consuming heat in the dissociation).
If that's what you think it is billed to be, you need to go back and look at it again much more carefully.
You deliberately misunderstood what I said. The reality is that there is insufficient information here - this is a research proposal (somebody wants a job!) and the process certainly isn't as simple as the diagram shown.
Ammonia production is indeed exothermic but at 400 degrees C and 200 atmospheres - to make ammonia from H2 & N2 at ambient temps and pressures takes energy and complexity - overall it is almost certainly endothermic, in this case some (but not all?) of the the required energy is coming from the sun - otherwise, like I say, we are saved - just hook up a turbine to every existing ammonia plant.
The reality of this proposed process is you have to store LOTS of hydrogen, if it is stored at ambient the problem is even worse!
Storing an energy source liquid like ammonia makes more sense than trying to store leaky hydrogen at ambient pressure in a closed system.
I'm getting confused by some of the comments here.
1. All of the required energy is coming from the sun - this is a concentrating solar thermal power plant, not a magic free energy source using ammonia.
2. The ammonia reactions simply store and release the energy captured from the sun.
3. I agree that there isn't a lot of detail around how the generation unit uses the reactions to control energy output (though perhaps if you read the patents and Dr Lovegrove's presentations you might become more enlightened). It isn't clear to me if this is more efficient than other energy storage schemes, though that seems to be what they are claiming.
4. This isn't a research proposal, it is a government funded pilot development by a private company that is being constructed. The research was done at ANU years ago.
5. I'm not promoting the company (as hinted at elsewhere) - it seems to be a small private company with no plans to list itself that I'm aware of. This plant isn't sufficient to base a commercial power company on anyway - they'll need to raise capital to expand further, but I doubt anyone will put money in until he concept is proven. I'm certainly not recommending anyone try to buy anything - just discussing a novel form of energy storage - maybe the pilot plant will be a success, maybe it won't - we'll have to wait and see.
Hmmm ... we'll see - the reason I am dubious is ammonia synthesis is one of the largest energy-consuming processes of the industrialized world requiring approximately 1% of the world's power production. From the diagram shown every ammonia plant should be able to generate more power than it consumes given a hydrogen supply - so, an easy question ... do they?
Yes, the ammonia reactions store and release energy - but it is the overall balance that matters.
The liquid and gas components are stored in the same container - do the math to see how big it will have to be to match an average coal fired power station output based on ten or so hours-a-day sunshine.
Pilot plants are R&D, this is an unproven concept, be especially dubious if the only people prepared to stump up the money are the Government.
You appear to be deliberately obtuse; no person of good faith could read the explanations elsewhere in this thread and continue with the counter-factual rants (trolls?) as you have above.
For the last time:
All of this was made abundantly clear elsewhere. If you cannot grasp it, the problem lies between your chair and your keyboard.
The problem that you can't grasp lies in the efficiency of the right hand side of the diagram, it needs to produce net-electrical-energy from the production of ammonia - the left hand side is just a (massive?) hydrogen generator, the cost of the hydrogen is the main consideration on that side.
It is far from clear that from the information given that the system is a net energy producer and even less clear that it would produce the energy at a profit or be competitive with alternatives like solar PV and batteries.
However, if it does actually work then finally we do have the silver BB and we are indeed saved!
What on earth are you talking about ????
Its a solar power plant - the sun produces all of the energy.
The plant uses (some) of this energy to convert ammonia to gases as a form of energy storage. These gases are then converted back to ammonia again as a mechanism for later releasing this energy.
Once you've fed the original supply of ammonia in thats it (produced using the usual methods).
You don't need to supply it with hydrogen at all.
Its not a silver bullet - its a solar plant with some energy storage.
Please read the article [edit] and these links again.
http://engnet.anu.edu.au/DEresearch/solarthermal/pages/pubs/ISESBeijingL...
http://engnet.anu.edu.au/DEresearch/solarthermal/pages/pubs/IJES06.pdf
http://engnet.anu.edu.au/DEresearch/solarthermal/pages/pubs/SolarEAmmoni...
http://engnet.anu.edu.au/DEresearch/solarthermal/pages/pubs/
They claim that the total conversion (sunlight to electricity) is 19%, compared to around 14% for some older CSP plants.
They also claim storage efficiency of 100%, piping losses of 3.9% and "transient effects" of 8% when calculating this number (see Table 1 on page 2 of the first link).
It would be interesting to se what the equivalent number is for Ausra's planned plant - if anyone feels like digging up the number, please post it...
NO - you don't understand what I'm saying.
There are two (linked) halves to this system - the hydrogen producing and storage part driven by the sun (it can be as inefficient as you want), and the electrical-energy production part driven by the production of ammonia.
This energy system is just like any other it is the net energy that is important, EROEI - so, the electrical energy must be sufficient to run the system AND create and maintain all the components of the system - by the looks of it the storage tank is the limiting factor, but Rankine cycle efficiencies are only around 60% or so and the solar panels track using some kind of flexible system so I would expect hydrogen leakage (especially if they are running at 200 atmospheres, if they aren't then you have compressor energy costs.)
In short, this has to be EROEI positive and profitable ... is it?
I guess that is why they are building a pilot plant!
You could have saved a lot of time and agro if you had just stated your final question clearly in the first place.
I appologise for not being more succinct - I have made a living from fixing complex systems that others couldn't fix - what is completely obvious to me is not obvious to others.
As I said earlier this is a classic R&D proposal - like all business decisions you have to take a risk, you won't know if it is profitable until you try to make and sell one.
People at the top of organisations are paid to deal with risk, people at the bottom are paid to deal with certainty.
The problem with all power from storage is the nature of the battery - gaseous hydrogen is not a very dense source of power.
However, if this does work profitably it is what we are looking for - big risk and big reward.
Why big reward ?
At the heart of this is a proposal for efficiently storing solar power using the ammonia disassociation process - any efficiency gain it provides over other energy storage schemes for solar thermal plants (whether it be molten salt, graphite or hot water) is what drives the economics of it as a solution, but its no major bullet or gamebreaker - just a potential increase in efficiency.
Patent here, which has some diagrams and explanations that have more detail than the other materials referenced :
http://www.google.com/patents?id=yl4wAAAAEBAJ&printsec=abstract&zoom=4&d...
Why big reward? - because the problem with power from the sun (or wind) is it is intermittent, but we need continuous output of electricty and this system provides continuous power if it works - as far as I know the other alternatives you suggest are theoretical and don't actually work profitably at present.
Also, because of the tracking solar it would even work at the South pole for a good part of the year - this is not just for hot desert areas.
However, looking briefly at the patent - as I feared, the solar absorbers do indeed track and work at high pressure, multistage compressors are recommended presumably using some of the electrical power.
Also, as I suspected, this is a very complex system - not nearly as simple as the original graphic, the complexity is caused by the need to work at high pressures and temperatures and be very energy efficient. The patent is a modification of an earlier one which recognised the huge cost of reliable high pressure piping - what they call "uneconomically high pressures".
Let's hope it is a net electricity producer and profitable.
In time, we'll see whose chair.
We've already seen, the question is self-recognition.
Like I say we'll see, but from where I'm sitting it applies to you - it is way too early for me to tell whether this will be a success, you think you know the future and I doubt you do.
This is a just a propsed new type of large, very complex, inefficient battery and time will tell if is cost effective.
BTW, I suggest you read the rules of this site, you are clearly a very obnoxious person.
<snort> This, from the person who persists in making clearly erroneous statements about the principles of operation (and things like hydrogen leakage) yet claims to judge the practicality.
One of the rules of civility is that you do not mis-state the claims of others. You've persistently made false claims regarding this proposal, mis-characterized statements by several people regarding the physics and chemistry of the process as claims about its economics, and other trollish behavior. In polite society, we call that lying and it is a serious faux pas.
I suffer neither fools nor liars gladly.
Ad Hominems indicate to me you don't understand the rules of this site or how the real world works - by calling me a liar and by your responses you clearly don't understand what I am saying or what the proposal is saying - you have such pre-conceived ideas that you ignore contrary arguments, actually it looks like you ignore individual words. One thing you most certainly are not is civil.
This is a proposal for BASE LOAD 24x7x52 power and it will have to compete economically with with other proven reliable, available sources such as coal, oil, gas, nuclear, conventional batteries and bagasse. If the product isn't price competitive the company will fail.
If you want a lie then to say this is powered by solar power is incorrect - in reality this proposal IS NOT solely powered by solar, it has a large amount of embedded fossil fuel in it's manufacture and daily maintenance/operation - this fossil fuel is the life blood of the system, without it it won't work.
The claim that ammonia is non toxic is a lie.
Contary to what you seem to believe this is about making ammonia from solar power - the idea is to produce continuous electricity.
Any leaks will occur at flexible high pressure parts of the system such as the tracking solar collectors, not the low pressure storage as you inferred I implied. The gasses can't be stored at high temps and pressure because that would drive the production of ammonia.
A large scale battery requires the stored energy to be energy dense (like coal, uranium or oil)- a mixture of hydrogen and nitrogen at atmospheric pressure is a very poor and uneconomic way of storing energy. The stored energy on this system is recovered using compressors and then the Rankine cycle which, at best, is 60% efficient - which means the energy storage of the gases is even less dense.
Oh, and BTW gas friges do not work at 200 atmospheres pressure or have flexible pipework!
No it isn't.
Solar power is never used to create ammonia in this scheme.
Please stop saying this.
Solar power is used to convert ammonia to its constituent gases.
These later recombine (reforming the ammonia) and releasing energy when they do so.
As for the economics, many (most ?) countries have clean energy targets - renewable energy projects don't need to be cost competitive with, say, brown coal - they need to be competitive with each other.
The tracking solar collectors don't contain ammonia or its disassociated gases - these components will never leak.
You really seem to have misunderstood this technology completely - I can't understand why you are so passionate about demonstrating this to everyone - please spend some time understanding how it actually works...
One last pass over your falsehoods and other nonsense (before your account is deleted, as I expect is imminent):
As do the nuclear plants you tout. As do wind farms. This is a red herring.
There is no fossil-derived input to the cycle. None. You have repeatedly claimed that e.g. replacement of hydrogen requires one, but you have zero basis for this claim. You're just making s**t up. If replacement hydrogen is required, it can be obtained renewably by reaction of bio-char with steam.
The claim was not made in the article. "environmentally benign" is the term used, and it is correct; spills of ammonia would cause no lasting damage. In this respect it is like methanol: toxic if consumed, but biodegradable and not a lasting hazard.
As with most of your other claims, you have it exactly backwards. This is about decomposing ammonia with solar energy, so that the energy of recombination can be harvested later.
You make several errors in two sentences:
All of this was made abundantly clear in the article itself: "the solar energy is stored in a chemical form at ambient temperature...."
Well, duh. This is an experimental pilot plant. The purpose is to test the concept. Magnetohydrodynamic generators were tested in the 1970's and didn't make the cut either. It was still worthwhile to look at them.
The storage would be at Haber process pressures, nowhere near ambient pressure.
You've proven your cluelessness, you can stop now.
You don't even know what "ad hominem" means. Claiming that you should be disregarded because you're a nitwit troll would be ad-hominem; going over your numerous falsehoods and logical errors and your refusal to acknowledge corrections and concluding that you're a nitwit troll (liar, etc.) is just logic.
WTF?
http://en.wikipedia.org/wiki/Haber_process#Ammonia_synthesis
Ammonia synthesis is endothermic! It takes high temperatures and very high pressures to do it. Nowhere it is explained where will those come from, and where the energy for those will come from.
It is exothermic with these reactants.
Chris
False ...
It takes high temperature and catalysis to get some of the N2 molecules to break. Because ammonia synthesis is only a little exothermic, this high temperature shifts the equilibrium most of the way towards (3 H2 + N2). High pressure shifts it the other way.
How shall driving gain nuclear cachet?
OK, upon additional research you seem to be correct (I was confused by the minus sign over there - was this a typo, or I didn't get the meaning of the variable?)
Even under these circumstances I am highly sceptical that a slightly exothermic reaction, requiring high pressures of the reactants can be utilized to produce power efficiently. The reaction itself requires multiple passes of the reactant over catalysts, which itself would take energy and reduce efficiency further... I don't know what their engeneering solution is, but I would guess that if there was efficient way to utilise this energy, then amonia producers would have been significant power producer too (given the amount of amonia envolved). I haven't heard of such thing.
Producing ammonia requires energy because you need to get the hydrogen. Thus, ammonia production is not a net energy producer. But, if you have the hydrogen available, it is. That is kind of the point of the system.
Why use ammonia? It's breakdown temperature is just about right for this type of solar concentrator. With the heat exchangers, things work out well thermodynamically and one can use pretty much arbitrarily large amounts of storage over indefinite time intervals. Thus, seasonal variations can be handled by this method. The conversion to chemical energy is pretty efficeint and the temperature for conversion back to heat is pretty good for steam so the overall efficiency looks pretty good. Slide 34 of this presentation covers this:
http://glenntodd.net/bze/ZeroEmissionsConference-SolarAnu-Lovegrove-1Jul...
John Holbrook is working out a solid state ammonia production method that may be beneficial in this context. I think there have been some contacts made already.
I am the one who first incorrectly said that the reaction of hydrogen with nitrogen is endothermic. I did not read the whole Wikipedia article that I quoted. Indeed, the process to produce ammonia is exothermic which is shown by the (-) sign for the enthalpy of the process. The sign of enthalpy is confusing to many. From the chemists standpoint, a negative sign for a process indicates that heat is evolved in the process and is thus lost to the system (for an explanation of this see: http://en.wikipedia.org/wiki/Exothermic_reaction ).
The heat given off in the process: N2(g) + 3H2(g) → 2NH3(g), ΔHo = -92.4 kJ/mol is not enough, however, to support the conversion reaction, in an industrial process, because of the high temperature(472 deg C)along with high pressure needed to optimize the reaction.
This is in contrast to, for example, the reaction of hydrogen with oxygen to produce water. In this case the reaction is highly exothermic and very nicely supports further reaction once initiated, in fact explosively for stoichiometric mixtures. You can store stoichiometric mixtures of hydrogen and oxygen for centuries in a closed container with no observable reaction occurring. This is because the energy of activation for this process is quite high and some energy(say a spark) must be input into the process to initiate the reaction. But watch out it takes place explosively once initiated. This is not the case in the ammonia synthesis - let the mixture return to room temperature and the reaction is to slow to be useful, even in presence of catalyst.
The Haber process requires large amounts of heat and high pressures(and catalyst) to obtain an acceptable reaction rate to form an equilibrium mixture with an optimized conversion to ammonia. Then the equilibrium mixture must be cooled to below the boiling point of ammonia (- 33.35 deg C) to separate liquid ammonia from the equilibrium mixture. This step too requires considerable amounts of energy. Even though unreacted hydrogen and nitrogen are then returned to the reactor, the process requires, overall, large amounts of energy input. Better catalysts than presently used will not change the equilibrium constant Kc at 472 deg C thus the process will remain energy intensive.
Thus the solar process for producing storable ammonia is flawed and I would not invest one penny in the concept. Essentially it is another perpetual motion scheme hidden in chemical sheep's clothing.
Parent | Parent subthread | Reply | Reply in new window | Start new thread
In the diagram, you can see that the ammonia is stored as a liquid at ambient temperature. It is therefore not kept at ambient pressure. Requiring that the product be cooled below the boiling point of ammonia at ambient pressure thus seems a little unreasonable. Simply cooling the reacted mixture by making steam in a heat exchanger might condense the ammonia. Oh wait....
Chris
You can rest easy mate.
You won't have to invest anything.
The Australian Government is footing the exorbitant bill for this trial... what was it again Gav? Oh, that's right... $Aus 7.4 million... currently approaching parity with $US.
Let's put that enormous waste of Australian tax payers money into perspective...
That's a few kilometres of highway.
Perhaps 40 minutes of Iraq war!*
Sure, it may not work. But for that price, I trust the boffins at ANU over your on the fly theorising, enough to say, let's build a prototype and see.
*@ 2 billion per week (if I did the calculation right)
Lord help us chem es. A negative enthalpy change is an exothermic reaction.
Sorry. Black holes from my chem years... too long time ago. (I confused it with the more straightforward way of signing the formula A + B = C +/- X kJ/mol).
What is missing from this discussion (aside from a sufficient grasp of chemical thermodynamics at times) is that this is just a variant of hydrogen storage, but with nitrogen as the abetting partner instead of oxygen.
2NH3 <--> N2 + H2
vs
2(H2O) <--> O2 + 2H2
In both cases, the recombination (reverse direction of that shown) is exothermic -- meaning that it gives off heat as it occurs. However, the formation of water is much more so (-241.8 kJ/mol) than ammonia (-46.1 kJ/mol).
In both cases, you are using sunlight to split the molecules into the respective diatomics which are then stored and later recombined to release energy. The only apparent advantage for the ammonia-based system is that you can store all the reactants and products together in one vessel.
But the reason for this underscores its biggest disadvantage. Kinetics. Nitrogen and hydrogen in a closed container won't recombine to form ammonia because the kinetics are very slow. This is, of course, the reason catalysts are necessary for ammonia synthesis. And, even with modern catalysts, high temperatures are required to speed things up. But that drives the equilibrium back towards the reactants (N2 and N2). This is due to the entropy term, as in:
dG = dH - TdS (pretend that the d's are deltas)
To compensate, high gas pressures are used. Even so, multiple passes over the catalyst is necessary. True, you eventually get back the added thermal energy, but getting a lot of heat out of the overall process seems rather problematic. Why go this route?
Hydrogen and Oxygen are much happier to recombine, needing only a little encouragement (add spark -- or rough edge on something -- BOOM). Better still, they can be recombined in a fuel cell.
Note that this ammonia scheme is distinct from STORING ENERGY IN AMMONIA, which involves energy input to create the ammonia and then releasing energy via combustion or a fuel cell.
As in any scheme where you are storing energy in the form of hydrogen gas, you are limited by the volume you can store and/or the energy it takes to compress it. All of the limitations with hydrogen storage still apply.
If there are more advantages to a closed loop system involving ammonia vs. water, they haven't mentioned them.
Well, the advantage is that sunlight cracks ammonia but not water so easily and, as you say, it stays cracked until you want it. Also, it uses collecting area about 1.4 times more efficiently than Solar Tres. You can read more about it here: http://glenntodd.net/bze/ZeroEmissionsConference-SolarAnu-Lovegrove-1Jul...
Chris
JB
The problem with water is the high temp (3500K) to get it to disassociate (see this paper)
The "beauty" of this approach is that the technology is relatively low risk, I'm sure that higher
efficiencies can be gained (solar stirling is still the benchmark IIRC) the only "non commercial" part is disassociating NH3 but that seems to occur a a lot lower temp and therefore doesn't have the material issues of water.
Also I think part of the "risk avoidance" strategy was to NOT use fuel cells. In the long term if direct solar water cracking can be made more efficient then this type of plant can move into higher efficiency modes ie Water -> H2 O2 -> Combustion -> Combined Cycle or Water -> H2 O2 -> Fuel Cell
Neven
Perhaps slightly OT, but has anyone any idea of the efficiency of using a zinc oxide to zinc reaction?
It seems from the discussion that elements of this ammonia scheme are problematic, so I wondered if this would do any better - concentrated solar could certainly be used, although I believe you would also need charcoal.
BG:
I'm puzzled by this scheme. The heat input from Sun seems to force ammonia to dissociate into N2 and H2, but they seem to remain mixed as they flow out of the reactor where the ammonia is broken down. What keeps this mixture stable? Can't the reverse reaction take place in the storage tank? I would think this is a serious problem because the reverse reaction, making ammonia from N2 and H2 is exothermic and, once started, will experience thermal run away. I don't doubt that they are making it work, but I do doubt that their marketing materials are telling the whole story. What are they *really* doing? Have you found a real chemical engineering discussion of this? Where?
I suspect it has to do with the heat exchangers at both the solar collector and the gas turbine in which stored energy is turned into mechanical energy. What is going on in these? What are the design criteria of these?
Also, in the pictures of the collectors, the reactor, which I suppose is at the focus of the parabola, is a very long distance from the parabola. This results in a rather low peak temperature for these collectors. Why do this? Again, I feel there is more to be said about what they are really doing.
What say you?
You've actually got to raise the temperature to 400C or thereabouts before the catalyst activates. At room temp the kinetics are pretty slow (in either direction).
OK. So there's catalyst in both the solar reactor and the Rankine cycle heater, and, with catalyst and a temperature of about 400C, the reaction is pretty much reversible. That implies that the input temperature to the gas turbine expander is limited to approx. 400C, which is not very high for a commercial grade Rankine cycle power generator. I wonder what their catalyst is, and how often it needs to be regenerated, and what they do to regenerate.
I think I'm not ready to invest in this.
Which if you followed this link (Haber and Bosch, above) leads to several sources of information, the first of which tells us that the catalyst for ammonia synthesis is basically iron oxide. Not much chance of that running out.
The dissociation of ammonia is achieved in products from Thermal Dynamix using nickel.
Which sort of begs the question of why different catalysts are used for dissociation and recombination, since catalysts -- as per the laws of thermodynamics -- must work equally well in both directions.
Either iron or nickel will work either way. Nickel costs a lot more but lasts longer, especially if you have temperature cycling. You can go cheap and change it out more often, or not cheap and not change it out.
I see someone is working on the separation problem http://pesn.com/2007/12/11/9500465_Ammonia_Fuel_Cells/
This fuel cell work uses a different chemical reaction in which water (H2O) is somehow involved. Water is released, but they don't say how it gets into the reaction. Do they use O2 an input, in addition to NH3?